|
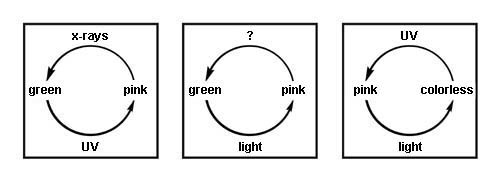
Early in the nineteenth century, a pink color was noted in some specimens of freshly exposed sodalite from Greenland. The pink faded rapidly to colorless once exposed to light. When placed in the dark for a time, the sodalite recovered the pink color. The alteration between pink and colorless could be repeated again and again. Minerals that are capable of such color change are said to be tenebrescent, from a Latin word referring to darkness. Another term sometimes applied is photochromic, a word that applies also to sunglasses, which change in color density with changes in illumination level.


Before exposure to daylight After exposure to daylight
Sodalite that shows this behavior has been given the variety name hackmanite. Since the pink color is quickly bleached by light, it is an unstable color in this mineral. That certain minerals can, without change of essential composition, lose or gain color when exposed to light is well known. Leaving the specimen in the dark from a few hours to several weeks may restore the pink color of hackmanite. Exposure to short- or long-wave ultraviolet will also restore color. Short-wave ultraviolet is the most effective for this purpose. The speed with which this is accomplished and the depth of color achieved varies from specimen to specimen.
In some specimens in whom the color centers are weak, long exposure to ultraviolet is required in order to produce a degree of pink color. In other specimens, exposure to short-wave ultraviolet will almost instantly produce a pink color. Particularly in the latter specimens, additional exposure to ultraviolet for several minutes to a few hours will produce a deep pink to raspberry-red color in which a weak blue color component is evident. If the specimen is now put in the dark, the deep pink or red color will remain, seemingly indefinitely. But visible light will quickly reverse this process and render the specimen colorless. Wavelengths between 480 and 720 nm, which encompasses the entire visible light spectrum except violet and blue, are effective in this. Hackmanite that responds in this way has been found at Bancroft; Mount Saint-Hilaire; Magnet cove; Ilimaussaq and Narssaq in Geenland; Langesundfjord in Norway and Khibina Massif at the Koala peninsula in Russia.
Most researchers appear to agree that F-Centers are the cause, or at least part of the cause, of reversible color in hackmanite. The term F-Centers comes form the German word Farbe, which means color. F-Centers are responsible for coloring a variety of minerals, including fluorite and barite. In hackmanite, it is proposed that some of the negatively charged chlorine atoms are missing. A negative electric charge is required at such vacancies to provide charge balance, and any free electrons in the vicinity become drawn to such vacancies and are trapped there. Such a trapped electron is the typical basis of an F-Center. It appears that this center in hackmanite absorbs green, yellow, and orange light and varying amounts of blue. When the hackmanite is seen in white light, red and some blue are returned to the eye, giving the hackmanite colors. It is likely that sulfur, as double negatively charged disulfide units, S22, is the source of the electrons. When ultraviolet is directed at the sodalite, it is absorbed at disulfide units. These each lose an electron and thus become S21- units. The free electrons wander to the chlorine vacancies where they are trapped coloring the mineral.
Model of color change in different minerals.
Spodumene (Kunzite)
Terlingua Calcite
Sodalite (Hackmanite)
More information and samples are available at Free Form Artists.

Hackmanite has a strong fluorescence.
This text is adapted by Hubert Heldner from an unknown source.
|